Introduction to Acid Rain
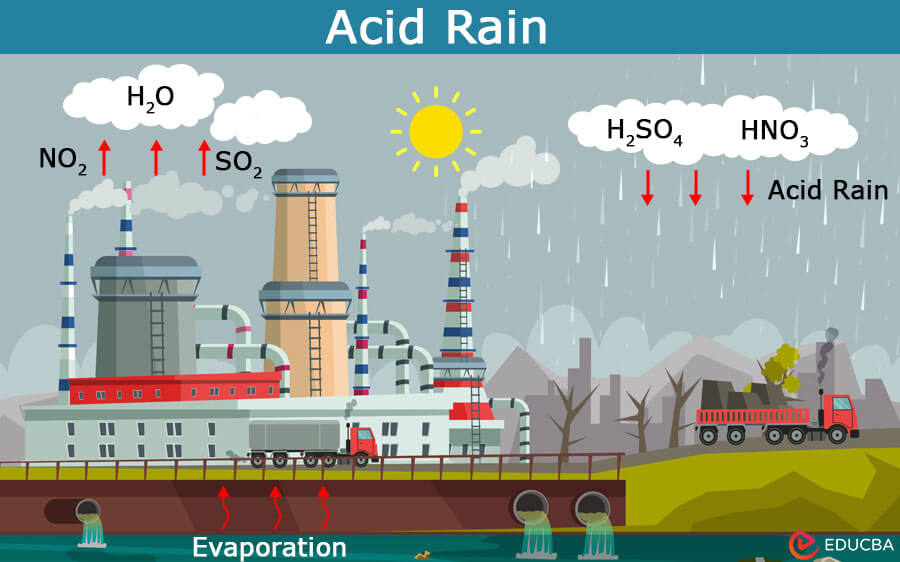
Acid rain, a term first coined in 1852 by Scottish chemist Robert Angus Smith, refers to precipitation with acidic components, such as sulfuric or nitric acid. This phenomenon results primarily from human activities, notably burning fossil fuels and industrial processes, releasing sulfur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere.
When these gases interact with water, oxygen, and other compounds, they create sulfuric and nitric acids, which subsequently descend to the earth’s surface as acid rain, snow, fog, or dust. For example, in the 20th century, acid rain caused significant damage to the forests of Europe and North America, particularly in regions downwind of major industrial areas.
Causes of Acid Rain
- Emissions from Fossil Fuel Combustion: Burning fossil fuels like coal, oil, and natural gas in power plants, factories, and automobiles emit sulfur dioxide (SO2) and nitrogen oxides (NOx) into the environment. These gases can react with water vapor, oxygen, and other substances to produce sulfuric acid and nitric acid, precipitating on Earth’s surface as acid rain.
- Industrial Activities: Industries that burn fossil fuels or produce chemicals can release large amounts of sulfur dioxide and nitrogen oxides into the air. Specific industrial processes, such as metal smelting and paper production, can also release these pollutants.
- Vehicle Emissions: Cars, trucks, and other vehicles that burn gasoline or diesel fuel emit nitrogen oxides and sulfur dioxide, contributing to the formation of acid rain.
- Agricultural Activities: Fertilizers that contain nitrogen compounds can release nitrogen oxides into the atmosphere. Livestock farming also produces ammonia, which can react with other pollutants in the air to form nitrogen oxides.
- Natural Sources: Although human activities are the main driver of acid rain, natural sources like volcanic eruptions and wildfires can also release sulfur dioxide and nitrogen oxides into the atmosphere. However, these natural sources typically contribute less to acid rain compared to human activities.
- Long-Distance Transport: Once released into the atmosphere, sulfur dioxide and nitrogen oxides can be carried long distances by wind and weather patterns before being deposited as acid rain. This means that acid rain can still affect areas far away from pollution sources.
- Acidic Deposition: In addition to acid rain, sulfur dioxide and nitrogen oxides can also be deposited on the Earth’s surface in the form of dry deposition, such as acidic particles and gases. This can occur when pollutants are not washed out of the air by precipitation.
- Chemical Reactions in the Atmosphere: Once in the atmosphere, sulfur dioxide and nitrogen oxides can undergo complex chemical reactions with other compounds, such as ozone and hydroxyl radicals, which can further enhance their transformation into sulfuric acid and nitric acid, contributing to the acidity of rainwater.
Environmental Impacts of Acid Rain
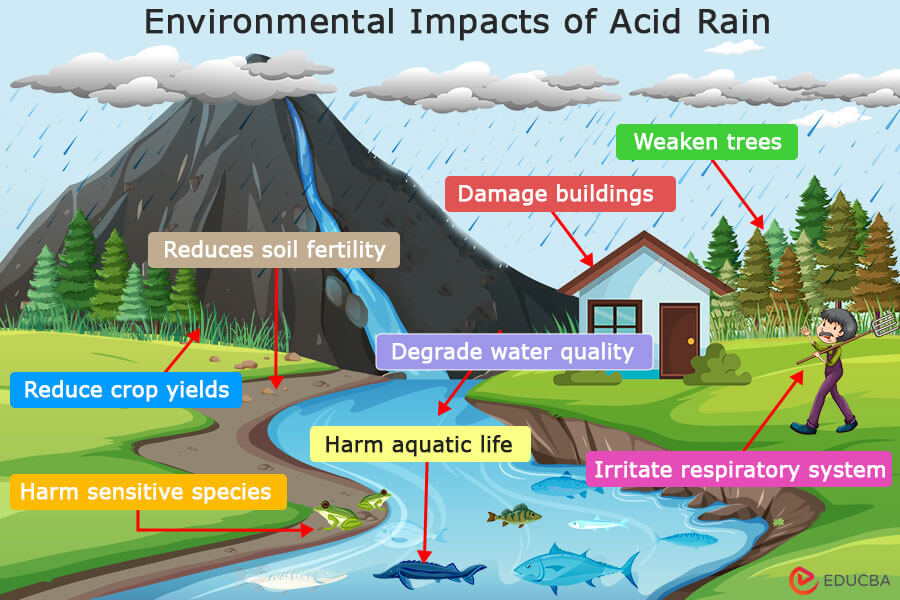
The environmental impacts of acid rain are significant and wide-ranging, affecting various ecosystems and natural resources. Some of the main environmental impacts are:
- Aquatic Ecosystems: Acid rain can lower the pH of lakes, rivers, and streams, making them more acidic. This can harm aquatic life, such as fish, frogs, and insects, as well as the plants and algae that form the base of the aquatic food chain. Acidification can also release harmful metals, such as aluminum, from the soil into water bodies, further impacting aquatic organisms.
- Soil and Plant Life: Acid rain has the potential to wash away nutrients like calcium and magnesium from the soil, reducing its fertility. This can impact the health of plants and trees, resulting in stunted growth, leaf damage, and heightened vulnerability to diseases and adverse weather conditions. Acidic soil can also affect the availability of nutrients to plants, further impacting ecosystem health.
- Forests: Acid rain can damage forests by leaching nutrients from the soil and weakening trees. This can make trees more vulnerable to diseases, insect infestations, and extreme weather events. In some cases, acid rain can contribute to forest decline, leading to ecosystem imbalances and biodiversity loss.
- Materials and Buildings: Acid rain can corrode and damage buildings, monuments, statues, and other structures made of limestone, marble, or other acid-sensitive materials. This can result in the deterioration of historical and cultural landmarks and infrastructure, such as bridges and roads.
- Human Health: Although direct exposure to acid rain is not a major health concern, the pollutants that cause it, like sulfur dioxide and nitrogen oxides, can have detrimental health impacts. These pollutants can irritate the respiratory system, exacerbate asthma and other respiratory conditions, and aid in forming fine particulate matter, which is linked to various health problems.
- Biodiversity: Acid rain can impact biodiversity by harming sensitive species and disrupting ecosystem balance. Some plant and animal species are more sensitive to changes in acidity and may decline or disappear in acidified environments, leading to shifts in species composition and ecosystem dynamics.
- Water Quality: Acid rain can degrade water quality by increasing the acidity of surface waters. This can affect the availability of clean drinking water and impact aquatic ecosystems that rely on balanced pH levels for survival.
- Economic Impacts: The environmental impacts of acid rain can have economic consequences, such as reduced crop yields, damage to forests and ecosystems that provide valuable services, and the cost of repairing and maintaining infrastructure damaged by acid rain.
Regional and Global Patterns
- Regional Variability: The distribution of acid rain is not uniform globally. Regions with high concentrations of industrial activity, such as urban areas and areas downwind of industrial sources, tend to experience more significant impacts than remote or less populated areas.
- Prevailing Wind Patterns: Wind patterns play a significant role in transporting pollutants that contribute to acid rain. Winds can carry pollutants emitted in one region over long distances before depositing them as acid rain, impacting areas far from their original source.
- Mountainous Areas: Mountainous regions can experience higher levels of acid deposition due to orographic lifting, which forces air masses to rise over mountains, causing increased precipitation and deposition of pollutants.
- Coastal Regions: Acid rain can impact coastal areas, especially those downwind of industrial or urban areas. The proximity to the ocean can also influence the acidity of rainwater due to interactions with sea salt aerosols.
- Global Transport: Although the impacts of acid rain are most noticeable at the regional level, the pollutants that cause acid rain can be transported globally through the atmosphere. This means that even areas far from major pollution sources can be affected by acid deposition.
- Acidification Hotspots: Certain regions may be considered hotspots for acid rain and acid deposition. They experience higher levels of acidity due to local sources of pollution, unique meteorological conditions, or geographical features that enhance the accumulation of pollutants.
- Seasonal Variations: The occurrence of acid rain can vary seasonally, with higher levels of acidity typically observed during periods of increased precipitation, such as spring and summer. This is because wet deposition, where pollutants are removed from the atmosphere by rain, is a primary mechanism of acid deposition.
- Long-Term Trends: Despite considerable efforts to curb sulfur dioxide and nitrogen oxide emissions, the long-term trends in acid rain deposition can fluctuate depending on factors such as economic development, regulatory policies, and technological progress. Monitoring and research continue to be important for understanding and addressing the global patterns of acid rain.
Mitigation and Solutions
- Legislative and Regulatory Measures: Governments can implement laws and regulations to limit the emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) from sources such as power plants, industrial facilities, and vehicles. For example, the Clean Air Act in the United States has significantly reduced acid rain-causing pollutants.
- Technological Solutions: Flue gas desulfurization (FGD) and selective catalytic reduction (SCR) technologies can be implemented in power plants and industrial facilities to lower sulfur dioxide (SO2) and nitrogen oxide (NOx) emissions. These technologies can help alleviate the effects of acid rain by decreasing the quantity of pollutants discharged into the atmosphere.
- Alternative Energy Sources: Shifting to renewable energy sources like solar, wind, and hydropower can diminish reliance on fossil fuels and lower emissions of pollutants that contribute to acid rain.
- Fuel Switching: Switching to cleaner fuels, such as natural gas, can also help reduce SO2 and NOx emissions and mitigate the impacts of acid rain.
- International Cooperation and Agreements: Given that acid rain can travel across national boundaries, international cooperation is crucial to address this problem effectively. Agreements such as the Convention on Long-Range Transboundary Air Pollution (LRTAP) aim to reduce air pollution and acid deposition across Europe and North America.
- Acid Rain Monitoring and Research: Continued monitoring and research are crucial for understanding the causes and impacts of acid rain and developing effective mitigation strategies. This includes monitoring rainwater’s acidity, studying its effects on ecosystems, and evaluating the effectiveness of mitigation measures.
- Public Awareness and Education: Educating the public about the causes and impacts of acid rain can help raise awareness and promote actions to reduce emissions and mitigate its effects. Citizen science initiatives can facilitate the monitoring of acid rain and its impacts.
- Ecosystem Restoration: In areas where acid rain has damaged ecosystems, restoration efforts can help restore biodiversity and ecosystem function. These efforts can include liming to neutralize acidity in soil and water, reforestation, and habitat restoration for affected species.
Case Studies
1. United States Acid Rain Program (ARP):
- Background: Implemented in 1990 under the Clean Air Act, the ARP aimed to reduce sulfur dioxide (SO2) and nitrogen oxide (NOx) emissions from power plants in the United States.
- Implementation: The program introduced a cap-and-trade system, setting a limit on the total amount of SO2 and NOx that participating utilities could emit. Utilities granted emission allowances, which they could trade with other utilities.
- Results: The ARP significantly reduced SO2 and NOx emissions, exceeding the program’s goals. By 2010, SO2 emissions had decreased by 56% and NOx emissions by 52% compared to 1990 levels. These reductions contributed to improvements in air quality and reductions in acid rain.
- Impact: The Acid Rain Program (ARP) showcased the efficacy of market-based strategies in curbing air pollution and served as a blueprint for comparable initiatives in other countries.
2. European Union Emissions Trading System (EU ETS)
- Background: Established in 2005, the EU ETS is the world’s largest cap-and-trade system for greenhouse gas emissions, including those contributing to acid rain.
- Implementation: The EU Emissions Trading System (ETS) encompasses over 11,000 power plants and industrial facilities within the EU and establishes a limit on the total emissions permitted. Companies receive allowances for emissions, which they can trade with other companies.
- Results: The EU ETS has significantly reduced emissions of acid rain-causing pollutants, with emissions falling by 9% between 2005 and 2019. The system has also helped drive investment in cleaner technologies and renewable energy sources.
- Impact: The EU ETS has demonstrated the feasibility of a large-scale cap-and-trade system for reducing emissions and has played a key role in Europe’s efforts to combat acid rain and climate change.
3. Canada’s Acid Rain Program
- Background: Canada implemented a cap-and-trade program in the 1990s to reduce emissions of SO2 from significant industrial sources.
- Implementation: The program set targets for emissions reductions and allowed companies to trade emission credits. Companies that reduced their emissions below their allocated allowances could sell excess credits to other companies.
- Results: The program led to significant reductions in SO2 emissions, with Canada achieving its target of reducing emissions by 50% below 1980 levels by 2000.
- Impact: Canada’s program demonstrated the effectiveness of market-based approaches in reducing emissions and improving air quality. It also served as a model for other countries seeking to implement similar programs.
Future Outlook
- Continued Emission Reductions: Efforts to reduce sulfur dioxide (SO2) and nitrogen oxide (NOx) emissions are expected to continue, leading to further improvements in air quality and reductions in acid rain deposition. This includes implementing stricter emissions standards for power plants and industrial facilities.
- Global Action: International cooperation will remain crucial in addressing the global issue of acid rain. Coordination of efforts to cut emissions and lessen the effects of acid rain globally will require continued involvement in agreements like the Paris Agreement and the Convention on Long-Range Transboundary Air Pollution (LRTAP).
- Adaptation Strategies: In regions where acidification has already occurred, adaptation strategies may be needed to help ecosystems and communities cope with the long-term effects of acid rain. These strategies could include liming lakes and streams, reforestation efforts, and habitat restoration for affected species.
- Monitoring and Research: Continued monitoring and research will be essential for tracking the effectiveness of mitigation measures, understanding the impacts of acid rain on ecosystems and human health, and identifying emerging threats. This will help inform future policy decisions and adaptation strategies.
- Climate Change Impacts: Climate change could impact the future of acid rain, as shifts in temperature and precipitation patterns may alter the creation and deposition of pollutants that cause acid rain. Therefore, efforts to mitigate climate change can also have co-benefits for reducing acid rain deposition.
- Public Awareness and Education: Continued public awareness and education efforts will be necessary for maintaining support for emissions reductions and other measures to address acid rain. Engaging individuals in citizen science and environmental stewardship can empower communities.
Conclusion
Despite significant progress in reducing SO2 and NOx emissions and mitigating acid rain, it remains a global environmental challenge. We need to continue efforts to reduce emissions further, protect ecosystems, and safeguard human health. International cooperation and agreements will be essential in addressing the transboundary nature of acid rain. Monitoring, research, and public awareness are crucial for informing policy decisions and fostering sustainable practices. By working together, we can continue to make strides toward a cleaner, healthier environment for future generations.